Laboratory Overview
Our laboratory focuses on advancing optical and histological methodologies for 3D characterization of tissue microarchitecture, vasculature, and innervation. By applying optical clearing methods, we render tissues—such as mouse and human intestine, pancreas, liver, and kidney—transparent, enabling detailed 3D visualization of fluorescently labeled structures. A key aspect of our research is integrating modern 3D histology (fluorescence and transmitted light imaging) with classic techniques, such as H&E and IHC staining, to enable multiplexed analysis of tissues in both health and disease.
==================================
New in 2023: Antifade technology for 3D & super-resolution imaging in high-n polymer
==================================
Our team recently developed a novel antifade method for 3D and super-resolution imaging, compatible with 3D Airyscan, STED, Lattice SIM, and NSPARC. This method uses solid high-refractive-index (high-n) polymers as an alternative to conventional immersion-based tissue clearing techniques.
Rather than using high-n liquids, we embed fluorescently labeled tissues in a solid, acrylamide-based high-n polymer through photo-polymerization. Much like tissue solidification with paraffin for H&E histology or resin for electron microscopy, this solvent-free environment is optimized for clinical tissue analysis.
By limiting oxygen diffusion into the specimen, it significantly reduces photobleaching and minimizes signal loss during imaging, while providing extended shelf life for long-term storage, transport, and repeated fluorescence imaging.
==================================
Keywords: 3D histology; human pancreas early change/lesion; human pancreas & liver 3D neurohistology; high-n polymer, solid-state tissue clearing; antifade 3D & super-resolution imaging; 1080p HD video; 3D Airyscan; STED; Lattice SIM; NSPARC; 3D super-resolution islet imaging
==================================
Our laboratory focuses on advancing optical and histological methodologies for 3D characterization of tissue microarchitecture, vasculature, and innervation. By applying optical clearing methods, we render tissues—such as mouse and human intestine, pancreas, liver, and kidney—transparent, enabling detailed 3D visualization of fluorescently labeled structures. A key aspect of our research is integrating modern 3D histology (fluorescence and transmitted light imaging) with classic techniques, such as H&E and IHC staining, to enable multiplexed analysis of tissues in both health and disease.
==================================
New in 2023: Antifade technology for 3D & super-resolution imaging in high-n polymer
==================================
Our team recently developed a novel antifade method for 3D and super-resolution imaging, compatible with 3D Airyscan, STED, Lattice SIM, and NSPARC. This method uses solid high-refractive-index (high-n) polymers as an alternative to conventional immersion-based tissue clearing techniques.
Rather than using high-n liquids, we embed fluorescently labeled tissues in a solid, acrylamide-based high-n polymer through photo-polymerization. Much like tissue solidification with paraffin for H&E histology or resin for electron microscopy, this solvent-free environment is optimized for clinical tissue analysis.
By limiting oxygen diffusion into the specimen, it significantly reduces photobleaching and minimizes signal loss during imaging, while providing extended shelf life for long-term storage, transport, and repeated fluorescence imaging.
==================================
Keywords: 3D histology; human pancreas early change/lesion; human pancreas & liver 3D neurohistology; high-n polymer, solid-state tissue clearing; antifade 3D & super-resolution imaging; 1080p HD video; 3D Airyscan; STED; Lattice SIM; NSPARC; 3D super-resolution islet imaging
==================================
1080pHD video: 3D/Airyscan super-resolution imaging of human intrapancreatic ganglion in high-n polymer
Toward a 3D new world of modern histology
3D images are essential for the study of tissue networks to characterize their morphologies in space. We establish tissue labeling and microscopic tools for high-resolution 3D imaging of mouse and human tissues.
Our featured cover images and illustrations
Integration of classic and in-depth tissue imaging (demonstration -- human pancreatic duct lesion)
Team and collaborators
|
PI: Dr. Shiue-Cheng (Tony) Tang; Dr. Pankaj J. Pasricha; Dr. Yuan-Chiang Chung; Dr. Chia-Ning Shen; Dr. Michael German; Dr. Chester Chamberlain; Dr. Luc Baeyens; David Scheel; Dr. Yu-Wen Tien; Dr. Yung-Ming Jeng; Dr. Chih-Yuan Lee; Dr. Shien-Tung Pan; Dr. Ming-Yin Shen; Dr. Yung-Chi Hou, Dr. Tsung-Lin Yang; Dr. Jyuhn-Huarng Juang; Dr. Ya-Yuan Fu; Dr. Tzu-En Hua; Dr. Pei-Yu Lin; M.Sc. Yu-Chen Chiu; Dr. Yuan-An Liu; M.Sc. Shih-Jung Peng; M.Sc. Chia-Tung Hsu; Dr. Chien-Chang Huang; Dr. Hung-Jen Chien; M.Sc. Ya-Hsien Chou; Dr. Fu-Ting Hsiao; M.Sc. Mei-Hsin Chung; M.Sc. Tzu-Hui Huang; Dr. Li-Wen Lo; Dr. Chien-Chia Chen
|
At the Movies: 3-D Technology & Gastrointestinal Histology |
|
|
|
|
About the PI
Shiue-Cheng (Tony) Tang, Ph.D.
sctang@life.nthu.edu.tw
Department of Medical Science
Institute of Biotechnology
National Tsing Hua University
101, Sec. 2, Kuang Fu Rd., Hsinchu, Taiwan, 30013
Tel: 886-3-574-2465
2017 American Gastroenterological Association (AGA) Fellow
2015 Visiting Scholar, Diabetes Center, UCSF
2012-present
Professor, National Tsing Hua University, Institute of Biotechnology
Professor, National Tsing Hua University, Department of Medical Science
Professor, National Tsing Hua University, Department of Chemical Eng (joint appointment)
2008-2011
Associate Professor, National Tsing Hua University, Taiwan, Dept. of Chemical Eng
2005-2008
Assistant Professor, National Tsing Hua University, Taiwan, Dept. of Chemical Eng
2005 Jan-Jul
Assistant Professor, Nanyang Technological University, Singapore, Div. of Chemical & Biomolecular Engineering, School of Chemical & Biomedical Engineering
2004
Postdoctoral Training, Pediatric Gastroenterology, Stanford University School of Medicine
1998-2003
PhD Training, Chemical and Biomolecular Engineering, Georgia Institute of Technology
Our publications on 3-D histology
Highlighted by Nature Reviews Gastroenterology & Hepatology 8: 600; “Improving 3D imaging of the enteric nervous system” https://www.nature.com/articles/nrgastro.2011.167
8. Liu YA, Chung YC, Pan ST, Hou YC, Peng SJ, Pasricha PJ, and Tang SC. 3-D illustration of network orientations of interstitial cells of Cajal subgroups in human colon as revealed by deep-tissue imaging with optical clearing. American Journal of Physiology - Gastrointestinal and Liver Physiology. 302:G1099-G1110, 2012. Selected as featured article: "3-D illustration of ICC network orientations"
Accompanied by a commentary: “Islet nerves in focus – defining their neurobiological and clinical role” https://pubmed.ncbi.nlm.nih.gov/23001378
|
15. Hsu CT and Tang SC. Nature Reviews Endocrinology, Cover image 2014 Jan-Dec: Mouse islet Schwann cell network. Winner of 2014 Nature Reviews cover image competition. https://www.nature.com/nrendo/volumes/10/issues/1
|
|
18. Peng SJ and Tang SC*. Nature Reviews Nephrology, Cover image 2015 Jan-Dec, all twelve issues: Projection of mouse renal pericytes and their association with glomeruli. https://www.nature.com/nrneph/volumes/11/issues/12
|
|
Nestin-GFP mouse showing the nestin-positive cell network in the small intestine. The nestin-GFP (green) cells form a network in the longitudinal muscle-myenteric plexus layer, submucosal layer, and also inside the villi. Some nestin-GFP–expressing cells are present around the vasculature, visualized using DiD vessel painting technique (cyan).
|
23. Kulkarni S, Micci MA, Leser J, Shin C, Tang SC, Fu YY, Liu L, Li Q, Saha M, Li C, Enikolopov G, Becker L, Rakhilin N, Anderson M, Shen X, Dong X, Butte MJ, Song H, Southard-Smith EM, Kapur RP, Bogunovic M, Pasricha PJ. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci USA,114: E3709-E3718, 2017. (Contribution: application of 3-D histology to investigate enteric nervous system)
|
|
24. Tang SC*, Peng SJ, Baeyens L, and German MS. Nature Reviews Endocrinology, Cover image 2017 Jan-Dec: Human pancreatic microenvironment in type 2 diabetes mellitus. Winner of 2017 Nature Reviews cover image competition. www.nature.com/nrendo/volumes/13/issues/1
|
|
28. Peng SJ and Tang SC*. Nature Reviews Endocrinology, Cover image 2019 Jan-Dec: Peri-islet vasodilation and lymphocytic infiltration in experimental type 1 diabetes mellitus. Winner of 2019 Nature Reviews cover image competition. www.nature.com/nrendo/volumes/15/issues/1
|
|
29. Chien HJ, Chiang TC, Peng SJ, Chung MH, Chou YH, Lee CY, Jeng YM, Tien YW, and Tang SC*. Human pancreatic afferent and efferent nerves: mapping and 3-D illustration of exocrine, endocrine, and adipose innervation. American Journal of Physiology - Gastrointestinal and Liver Physiology, 317: G694–G706, 2019.
|
|
31. Lin CY and Tang SC*. Nature Reviews Nephrology, Cover image 2020 Jan-Dec, all twelve issues: In-depth fluorescence imaging of renal blood vessels. Winner of 2020 Nature Reviews cover image competition. www.nature.com/collections/fifigibgci ; www.nature.com/nrneph/volumes/16/issues/1
|
|
|
In-depth imaging of optically cleared NOD mouse pancreas with insulitis. Overlay of transmitted light and fluorescence signals identifies the Lyve1+ lymph node (filled with CD3+ T lymphocytes and surrounded by fats, image at the top) and locations of insulitic islets. Islets with insulitis are shown with blood vessels (red), lymphatic vessels (magenta), and nuclei (white). CD3+ T lymphocytes are identified around the islets and congregated in the lymphatic vessels.
|
35. Shiue-Cheng Tang. 2021 US Patent 11,009,433: Composition and method for solid-state tissue clearing. https://uspto.report/patent/grant/11,009,433
|
36. Chung MH, Chien HJ, Peng SJ, Chou YH, Chiang TC, Chang HP, Lee CY, Chen CC, Jeng YM, Tien YW*, Tang SC*. Multimodal 3-D/2-D human islet and duct imaging in exocrine and endocrine lesion environment: associated pancreas tissue remodeling. American Journal of Physiology - Endocrinology and Metabolism, 323:E354-E365, 2022.
|
|
37. Hsiao FT, Chien HJ, Chou YH, Peng SJ, Huang TH, Lo LW, Sheng CN, Chang HP, Lee CY, Chen CC, Jeng YM, Tien YW, Tang SC*. Transparent tissue in solid state for solvent-free and antifade 3D imaging. Nature Communications, 14:3395, 2023. https://www.nature.com/articles/s41467-023-39082-4
|
|
38. Chen CC, Peng SJ, Chou YH, Lee CY, Lee PH, Hu RH, Ho MC, Chung MH, Hsiao FT, Tien YW, Tang SC*. Human liver afferent and efferent nerves revealed by 3-D/Airyscan super-resolution imaging. American Journal of Physiology - Endocrinology & Metabolism, 326:E107-E123, 2024. https://journals.physiology.org/doi/abs/10.1152/ajpendo.00205.2023
|
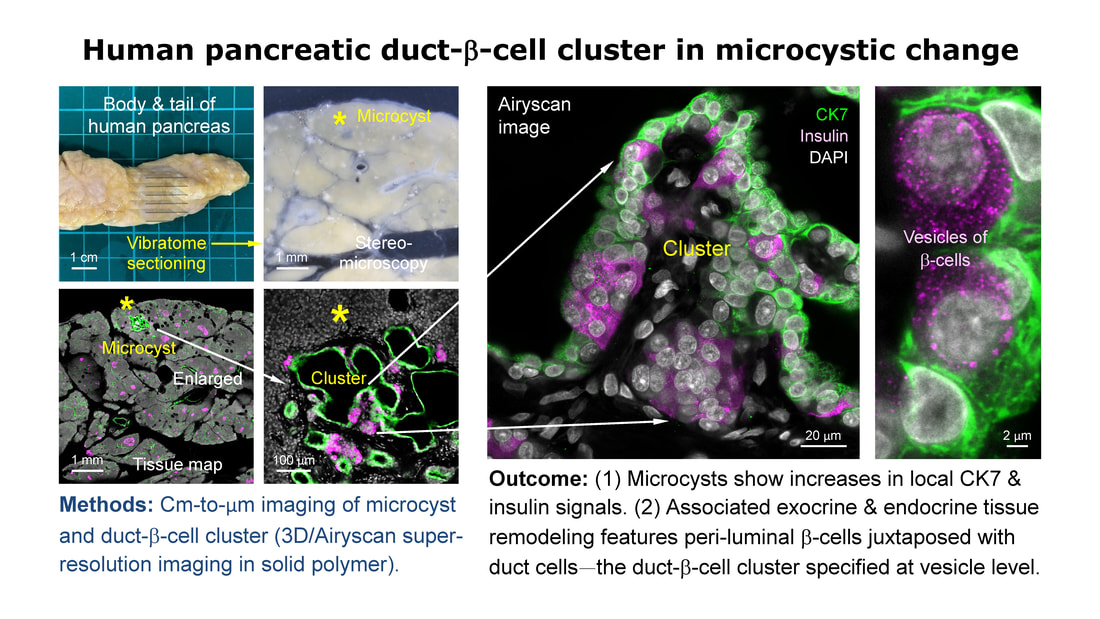
39. Lee CY, Kuo TC, Chou YH, Peng SJ, Hsiao FT, Chung MH, Lo LW, Shen CN, Chien HJ, Chang HP, Chen CC, Jeng YM, Tien YW*, Tang SC*. 3D imaging resolves human pancreatic duct-beta-cell clusters during cystic change. Diabetes, 74:734-748, 2025. https://pubmed.ncbi.nlm.nih.gov/39787388/
Accompanied by a commentary: “Bend It Like Occam: Ductal Origin of New Islet Cells in Human Pancreas After Injury.” Diabetes, 74:682-684, 2025. https://pubmed.ncbi.nlm.nih.gov/40258166/
Accompanied by a commentary: “Bend It Like Occam: Ductal Origin of New Islet Cells in Human Pancreas After Injury.” Diabetes, 74:682-684, 2025. https://pubmed.ncbi.nlm.nih.gov/40258166/